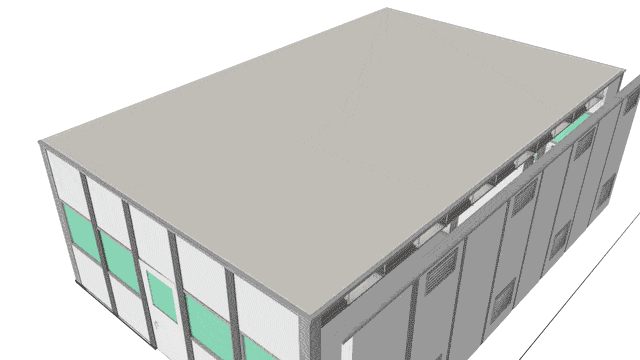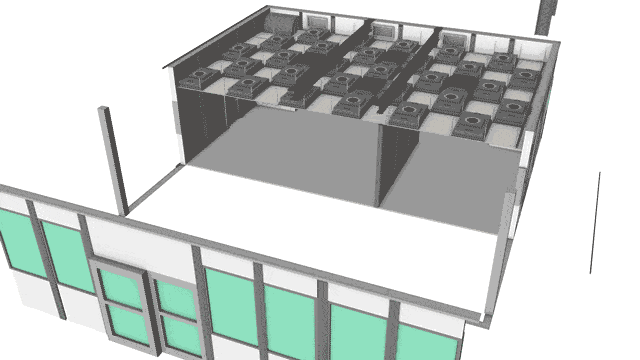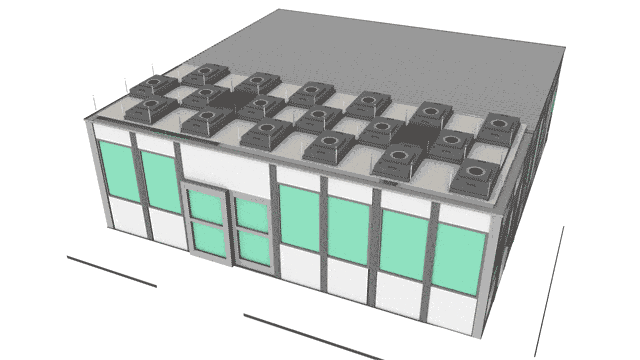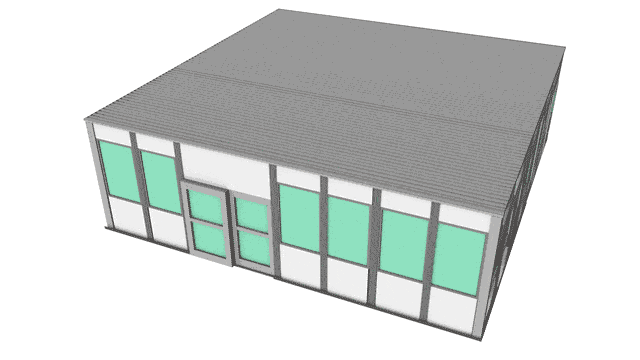
USP <797> Cleanroom Construction

Budget, facility size, existing structures, application, future proofing: these all affect how experts tackle operational temperature, humidity, HVAC, and particle control. Quick turnaround requires tight scheduling of plumbers, electricians, architects, engineers and handymen.
Once all the contractors go home, who do you call when particulate testing or a pharmacy board inspection reveals shortcomings? The most intimate details of your cleanrooms design and performance become strewn across dozens of manufacturers, engineers, facilities personnel, and installation teams. Why not consolidate expertise, installation, and final validation into a single point of contact and get it right the first time, guaranteed?
Compliance and Validation Testing is our Specialty
Our USP 797 cleanroom customers know that improperly designed control systems result in unnecessary contamination, mix-ups, and safety hazards. A CleanPro® system emphasizes performance under dynamic operation.
Because we pre-engineer your cleanroom for your specific compliance and application, we guarantee that our cleanrooms meet your state, federal, and organizational benchmarks.
We'll handle every part of the design and installation processes. Our customers rest easy because they know we've tested process parameters and function throughout an operation sequence. They enjoy less phone time, fewer emails, and minimal downtime. We guarantee a cleanroom that meets your safety and performance benchmarks.
 USP 797 Guidelines
USP 797 Guidelines
United States Pharmacopeia USP 797 took effect on January 1st, 2004 as a regulatory document which outlines procedures and environmental requirements for compounded sterile preparations (CSPs). Favorable outcomes in USP 797 cleanrooms also require proper laminar flow, workstation placement, operator technique, sanitation, and room air cleanliness.
USP 797 expands compounding guidelines for the preparation, storage, and handling of sterile drugs, both hazardous and non-hazardous. Learn more.
ISO 9001:2015 Certified
ISO 9001:2015 is an internationally recognized standard for Quality Management Systems (QMS). It is designed to provide companies with a set of principles that ensure a systematic approach to achieving customer satisfaction.
CleanPro® a division of Production Automation corporation, has implemented procedures to ensure a consistent standard of service for our customers, and customer feedback monitoring to uphold expectations between our vendors, warehouses, and the end-user. We continually conduct internal reviews to ensure that we are always improving.
Get Cleanroom Design Help at No Cost
The right solution starts with the right questions. CleanPro® expertise is always free. That means you get the benefit of a free quote that highlights the right solution at the lowest possible cost. CleanPro® grants the support of a cleanroom network that spans hundreds of manufacturers and in-stock warehouses across the Americas.